How Do Chemists Organize Information About Elements
The rows periods and columns groups organize elements according to similar properties. An element in an A group in the periodic table.

Pin On Periodic Table Of The Elements
The periodic table of elements arranges all of the known chemical elements in an informative array.

. Mendeleev arranged the elements in his periodic table in order of increasing atomic mass. In his system elements were grouped into triads. For example in hydrogen element 1 there is 1 proton in Helium 2 and in Oxygen 8.
The periodic table is a graphical collection of element data. Elements are arranged from left to right and top to bottom in order of increasing atomic number. Through development of quantum mechanics.
Each horizontal row on the periodic table is called a period. Each vertical column on the periodic table is called a group. When given a pair of elements in either the same series or group.
The number of protons is called the atomic number. Through application of the atomic theory O C. A triad is a set of three elements with similar properties.
Every atom of element 118 has 118 protons. The horizontal rows are called periods and the vertical columns are called groups. Be able to tell.
Your email address will not be published. A logical way to organize the elements. Chemists often specialize in a particular branch of the field.
Leave a Reply Cancel reply. It is a masterpiece of data visualization. Dobereiner 17801849 published a clas-sification system.
The chemical elements are arranged in order of increasing atomic number. One hundred fifty years after Russian chemist Dmitri Mendeleev published his system. Elements were now ordered by atomic number the number of positively charged particles called protons in the atomic nucleus rather than by atomic mass but still also by chemical similarities.
In the modern periodic table elements are arranged in order of increasing atomic number. The table lists the chemical elements in order of increasing atomic number which is the number of protons in an atom of an element. Scientists then came up with the idea that they could arrange the element by the atomic mass number.
The periodic table is an icon. Elements are listed in numerical order by atomic number. In their atoms the s.
Order generally coincides with increasing atomic mass. Get 1-on-1 help from an expert tutor now. Analytical chemists determine the structure composition and nature of substances by examining and identifying their various elements or compounds.
Most non-metals were gases and liquids brittle and poor conductors of heat and electricity. As a group these elements display a wide range of physical and chemical properties. Chemists used the properties of elements to sort them into groups.
Keeping this in view how do chemists organize information about. Elements are listed in numerical order by atomic number. Who was the first chemist to organize elements by atomic number.
Johann Wolfgang Döbereiner Antoine Lavoisier Henry Moseley John Newlands. In the modern periodic table elements are arranged in order of increasing atomic number name the three broad classes of elements. The following are examples of types of chemists.
- Shashank age 10 California USA. In 1829 a German chemist J. By grouping the elements on the periodic table.
Required fields are marked Comment Name Email. And its incredible design clearly shows the groups and trends among 118 different elements. Every element has its own unique atomic mass number because elements have different numbers of protons and neutrons.
Heres how it works. How do chemists organize information about elements. The periodic table brings order to information about the chemical elements.
Given an elements position on the periodic table be able to provide information about the elements electron configuration especially the outer energy level. In short the periodic table is more than just a table. The chemist that made and organized the periodic table was Mendeleev.
They also study the relationships and interactions among the parts of compounds. In an early attempt to organize the elements into a meaningful array German chemist Johann Döbereiner pointed out in 1817 that many of the known elements could be arranged by their similarities. Early chemists used the properties of elements to sort them into groups.
Mendeleev arranged the elements in his periodic table in order of increasing atomic mass. But chemists still cant agree on how to arrange it. How do chemists make new elements.
Every atom of hydrogen has 1 proton. Through use of the scientific method D. Three classes of elements are metals nonmetals and metalloids.
Which one has larger atoms which one has a higher first ionization energy which one has the higher electronegativity and which type of ion. The atomic number is the number of protons in an atom of that element. The rows are called periods.
How do chemists organize information about elements. There are two rows of elements found below the main body of. It helps chemists to understand why elements react as they do.
Its one of the most useful tools in a chemists repertoire. Until a new element is discovered the last element on the table is element number 118. An element is an atom whose nucleus includes a certain definite number of protons.
He also included groups and periods as groups contained the elements that had similar properties. The elements in Figure 61 formed one triad. So element number 1 hydrogen is the first element.
By grouping the elements on the periodic table B. He organized the periodic table of elements from increasing atomic number.
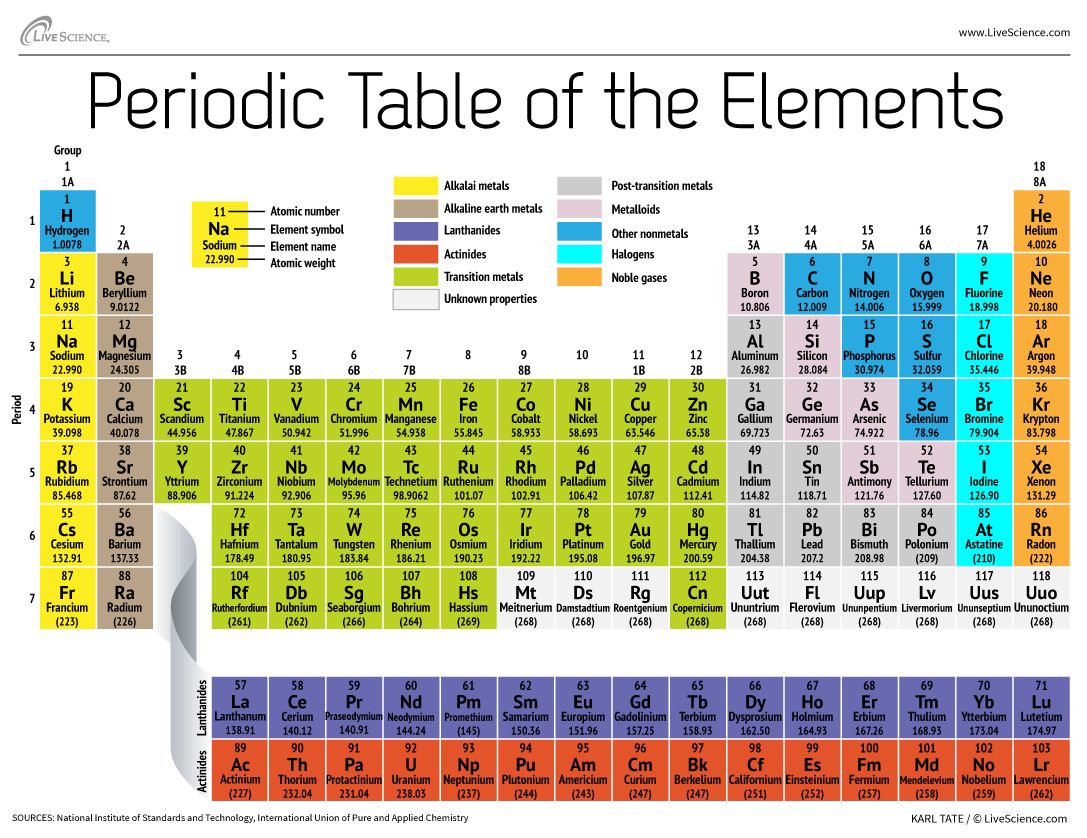
How The Periodic Table Groups The Elements Live Science

The Element Of Surprise Oh Fake Periodic Table Element Mug Etsy In 2022 Tea Mugs Mugs Funny Coffee Mugs

Elements And The Periodic Table Chem 1305 Introductory Chemistry
No comments for "How Do Chemists Organize Information About Elements"
Post a Comment